C3-Mobility – Selected project results
Find out more by clicking on the individual modules.
Work package A
Work package A completed: Analysis of the production of base product methanol based on hydrogen and CO2
Several of our partners have investigated methanol production paths from various CO2 sources. The work package was managed by Jülich Research Centre. bse Leipzig, process provider for electricity-based methanol plant modules (FlexMethanol), provided data for methanol synthesis. Methanol is the basis of all fuels examined in the C3 mobility project. The aim of work package A is therefore to evaluate the production of this raw material technically and energetically. For this purpose, three different CO2 sources, water electrolysis and methanol synthesis were analyzed, and the overall efficiency and the components required were determined. The results of work package A flow into the system evaluation and market launch strategies in work package E and will serve as the basis for the overall techno-economic evaluation of all fuels. For the production of methanol, hydrogen, based on electricity generated from renewable sources, and a sustainable CO2 source are required. The completed work package A therefore examined possible CO2 sources, different electrolysis variants and the CO2 -based synthesis of methanol.
Industrial exhaust gases, CO2 from biomass and direct CO2 separation from the air were identified as possible CO2 sources, see Figure 1. For industrial emissions, only process-related CO2 emissions were taken into account, e.g. in cement and steel production or in the chemical industry. The biogenic CO2 sources examined biogas production, bioethanol, waste-to-energy plants and sewage treatment plants. The direct separation of CO2 from the atmosphere (DAC) is possible with the aid of absorption and adsorption processes, the energy requirements and temperature levels of which were shown.
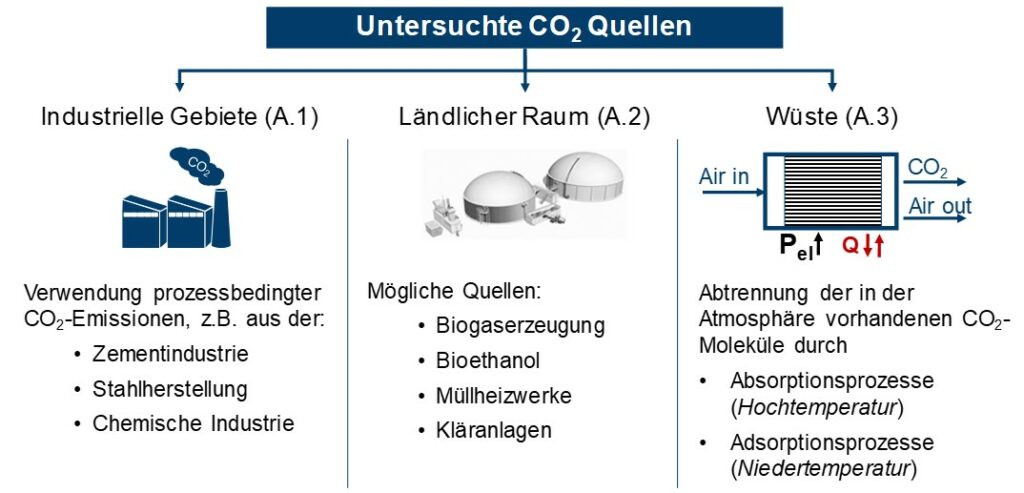
For the three CO2 sources, the existing separation technologies for the technical evaluation as well as their respective energy expenditure were determined as target values. The energy consumption has an impact on the overall efficiency of methanol production. In addition to classic separation processes, such as amine washing (MEA) or pressure swing absorption, the possibilities of membrane separation and the chilled ammonia process for industrial exhaust gases and biogenic CO2 sources were also examined.
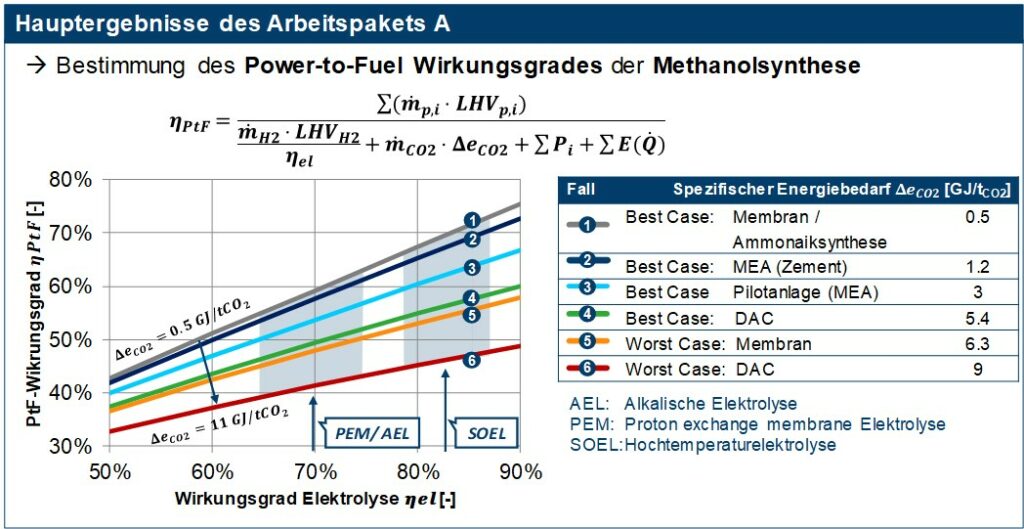
For the evaluation of the hydrogen production, the current and future forecast development status of the three common electrolysis variants alkaline, PEM and high-temperature electrolysis was shown. These differ in the operating temperatures, charge carriers, electrolytes, catalysts used and in technological maturity. The methanol synthesis based on H2 and CO2 is in addition to the classic production a process that is now also commercially available from synthesis gas. Methanol synthesis is characterized by high carbon efficiency, high system efficiency, etc. through the pressure adjustment electrolysis with methanol synthesis and integration options in the respective locations. Measures to adapt the methanol synthesis to modern requirements have an impact on profitability. Essential here is flexibility as a regulatory requirement for the product and generation of additional revenue potential, modularization with a reduction in investment and operating costs, implementation of resource efficiency and CO2 reduction. In the last part of work package A, the synthesis was analyzed in terms of process technology and the required starting materials (H2 and CO2) as well as the consumable of electricity and steam were determined. In addition, the process analysis provides the input data for the techno-economic analysis of all fuels, which was also carried out in the C3 mobility project, in the form of a detailed list of components for the production of methanol. With the results of work package A, the map shown in Figure 2 for the power-to-fuel (PtF) efficiency of methanol synthesis was created. This PtF efficiency describes the ratio of the energy bound in the synthetic fuel to the energy expenditure of its production, i.e. the production of hydrogen (electrolysis), the provision of the required carbon dioxide, and other consumables such as electricity or steam. The results in Figure 2 show that both the type of electrolysis and the CO2 source have a significant influence on the overall efficiency of the methanol synthesis. PtF efficiencies of 50-60% can already be achieved today with established CO2 separation processes available on the market, such as MEA amine washing and electrolysers (AEL / PEM). With a forecast development in electrolysis due to increasing demand, PtF efficiencies of over 60% will also be possible in the future.
Work package B3
15.400 liters of synthetic gasoline generated from green Methanol
In the gas synthesis plant at the TU Bergakademie Freiberg converted for C3-Mobility, high-octane petrol is produced from green methanol. Around 15,400 liters have already been made available for the project partners.
The joint research project C3-Mobility focuses on the production, characterization and application of modern fuels and blend components for the development and demonstration of new ways into the CO2 neutral mobility of the future. The use of regeneratively produced petrol based on methanol is particularly advantageous for this.
The C3-Mobility consortium requires sufficient amounts of synthetic gasoline to enable a wide range of studies on fuel properties and material compatibility, tests in engine applications and testing in gasoline and flex-fuel cars. To this end, the two professorships in Reaction Engineering (RT) and Energy Process Engineering and Thermal Waste Treatment (EVT) at the TU Bergakademie Freiberg are carrying out their joint sub-project, which comprises a volume of € 3.3 million and is fully funded by the BMWi. The RT professorship works on the development of advanced catalysts for gasoline synthesis, which includes kinetic studies, model creation and long-term tests. The project partner Chemieanlagenbau Chemnitz GmbH (CAC) also carries out catalyst tests and is in close contact with the RT professorship.
Since 2010, a large-scale gasoline synthesis plant based on CAC technology has been operated at the EVT professorship. In order to upgrade it for multi-week test campaigns for the production of high-octane gasoline of constant quality, it was converted to a new reactor concept. This is part of CAC’s current methanol-to-fuel technology, which will also be used to produce synthetic gasoline from methanol on an industrial scale in the future.

Figure 1: Assembly of a new gasoline synthesis reactor (May 2019)
In the one-year preparation phase after the start of the project, the new reactor concept was planned and implemented by CAC. On the part of the cooperating professorship EVT, approval procedures and TÜV tests were carried out, necessary repair and commissioning-preparatory work was carried out, raw material deliveries and product logistics were clarified and the product analysis accompanying the test was prepared.
At the beginning of October 2019, the first gasoline production phase started in the large-scale gasoline synthesis test facility of EVT. Particular attention was paid to determining the gasoline composition (detailed hydrocarbon analysis), which was promptly incorporated into the control and optimization of the process parameters. As a result of the four-week test campaign, a total of around 15,400 liters of high-octane gasoline were produced from “green methanol” (biomethanol, certified according to ISCC-EU). Special quality features are the low proportions of aromatic compounds via C9, especially the low Durol content, as well as a high oxidation stability. This gave the C3-Mobility consortium a first representative amount of fuel for fuel characterization and testing.

Figure 2: Steel containers (IBC) for petrol filling provided by project partner Shell

The second gasoline production phase is currently being prepared at the Professorship EVT. Based on the operational experience of the first test campaign, synthetic processes and test results become more material and economical key figures comprehensively evaluated and requirements of the C3-Mobility project partners for the product quality of the second batch incorporated into the test planning. Accordingly, a different catalyst was selected together with CAC.
It is currently being tested again at CAC in order to finally coordinate the setting values for the synthesis process up to the start of the test drive.
From August, a further 15 to 25 cubic meters of “green” gasoline of optimized quality will be produced in the large-scale gasoline synthesis test facility at EVT and made available to the project partners for their investigations.
Photos: Institute of Energy Process Engineering and Chemical Engineering at TU Bergakademie Freiberg
Work package C2
Increasing efficiency and reducing emissions on gasoline engines using synthetic fuels
Pleasing results come from work package C2. Under the leadership of the Institute for Combustion Engines (VKA) at RWTH Aachen University, ten fuel mixes with methanol, ethanol, iso-butanol and 2-butanol as well as methanol as a pure component were examined with regard to their suitability for use in car gasoline engines.
An oxygen-free gasoline with a research octane number (RON) of 94 served as the basic fuel for the thermodynamic investigations on a single-cylinder research engine with direct injection. The pure alcohol components were added to this basic fuel in proportions between 3% and 40% (v / v). A total of ten fuel mixtures with different properties were determined. The individual characteristics can be seen in Figure 1.
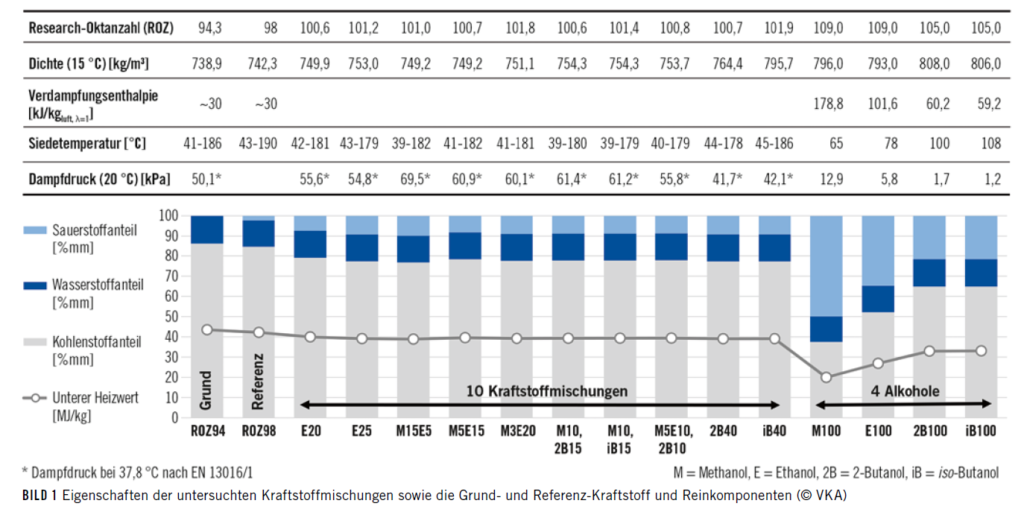
The defined mixtures as well as pure methanol and the reference fuel should then be considered in terms of efficiency and emissions. The basic investigations included stoichiometric partial and full load operation, charge dilution by means of exhaust gas recirculation (EGR) and excess air as well as catalyst heating and oil dilution.
Overall, the results are very promising: For example, pure methanol has been shown to have great potential for increasing efficiency and reducing NOx emissions. With the same compression ratio, methanol was 23.5% more efficient than the reference fuel RON98. The efficiency increase for fuel blends was 12.6%. By combining a high enthalpy of vaporization with a high laminar flame speed, methanol enables knock resistance and high combustion stability under high loads and cold start conditions.
In comparison, the blends with butanol and ethanol had a higher calorific value, but were less knock-resistant. The higher boiling temperature also triggered an increase in HC emissions under cold start conditions. With regard to EGR compatibility and lean-burn combustion, further efficiency advantages and an emission reduction with the fuel mixtures and pure components can be expected.
Cover Picture: FEV Europe GmbH, remaining pictures: vka
More Information
Christian Wouters
Research Associate
Gasoline engines Thermodynamics
Institute for Combustion Engines VKA
RWTH Aachen University
E-Mail: wouters@vka.rwth-aachen.de
Work package C7
1-octanol as a CO2-neutral fuel for commercial vehicle applications
Not only the passenger car sector but also the truck sector has committed itself to massive CO 2 reduction targets. High demands on range and refueling or charging times for these applications mean that renewable chemical energy sources – biofuels and e-fuels – are of particular importance. For this reason, FEV Europe is investigating, among other things, the medium-length chain alcohol 1-octanol as a possible energy source for commercial vehicle applications within the joint project Closed Carbon Cycle Mobility – Climate-neutral Fuels for the Traffic of the Future.
More Information