Work package A completed: Analysis of the production of base product methanol based on hydrogen and CO2
Several of our partners have investigated methanol production paths from various CO2 sources. The work package was managed by Jülich Research Centre. bse Leipzig, process provider for electricity-based methanol plant modules (FlexMethanol), provided data for methanol synthesis. Methanol is the basis of all fuels examined in the C3 mobility project. The aim of work package A is therefore to evaluate the production of this raw material technically and energetically. For this purpose, three different CO2 sources, water electrolysis and methanol synthesis were analyzed, and the overall efficiency and the components required were determined. The results of work package A flow into the system evaluation and market launch strategies in work package E and will serve as the basis for the overall techno-economic evaluation of all fuels. For the production of methanol, hydrogen, based on electricity generated from renewable sources, and a sustainable CO2 source are required. The completed work package A therefore examined possible CO2 sources, different electrolysis variants and the CO2 -based synthesis of methanol.
Industrial exhaust gases, CO2 from biomass and direct CO2 separation from the air were identified as possible CO2 sources, see Figure 1. For industrial emissions, only process-related CO2 emissions were taken into account, e.g. in cement and steel production or in the chemical industry. The biogenic CO2 sources examined biogas production, bioethanol, waste-to-energy plants and sewage treatment plants. The direct separation of CO2 from the atmosphere (DAC) is possible with the aid of absorption and adsorption processes, the energy requirements and temperature levels of which were shown.
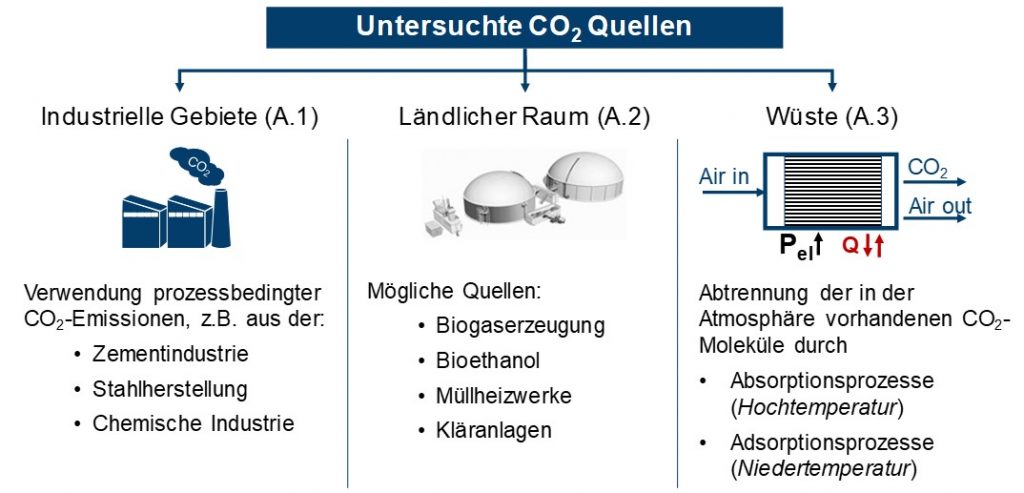
For the three CO2 sources, the existing separation technologies for the technical evaluation as well as their respective energy expenditure were determined as target values. The energy consumption has an impact on the overall efficiency of methanol production. In addition to classic separation processes, such as amine washing (MEA) or pressure swing absorption, the possibilities of membrane separation and the chilled ammonia process for industrial exhaust gases and biogenic CO2 sources were also examined.
<! – / wp: paragraph -> <! – wp: paragraph ->
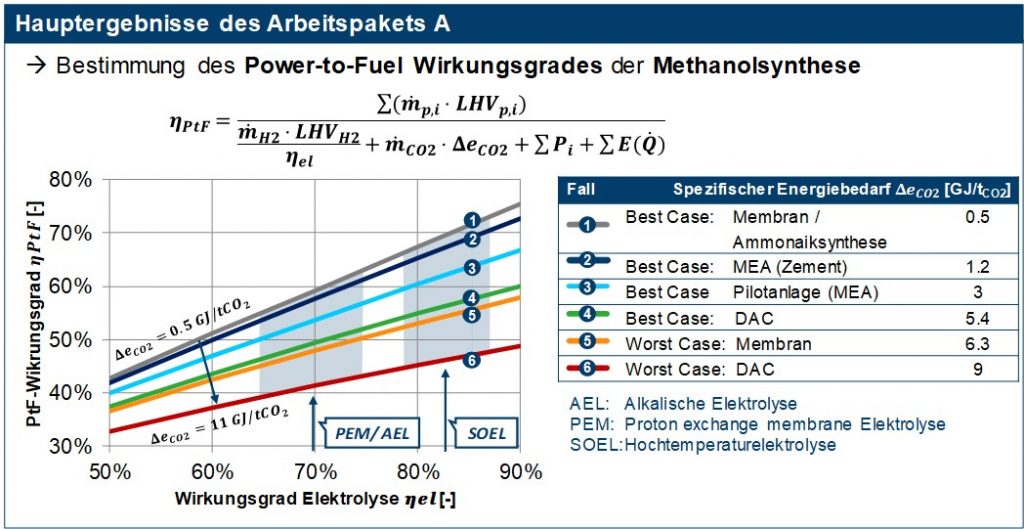
For the evaluation of the hydrogen production, the current and future forecast development status of the three common electrolysis variants alkaline, PEM and high-temperature electrolysis was shown. These differ in the operating temperatures, charge carriers, electrolytes, catalysts used and in technological maturity. The methanol synthesis based on H2 and CO2 is in addition to the classic production a process that is now also commercially available from synthesis gas. Methanol synthesis is characterized by high carbon efficiency, high system efficiency, etc. through the pressure adjustment electrolysis with methanol synthesis and integration options in the respective locations. Measures to adapt the methanol synthesis to modern requirements have an impact on profitability. Essential here is flexibility as a regulatory requirement for the product and generation of additional revenue potential, modularization with a reduction in investment and operating costs, implementation of resource efficiency and CO2 reduction. In the last part of work package A, the synthesis was analyzed in terms of process technology and the required starting materials (H2 and CO2) as well as the consumable of electricity and steam were determined. In addition, the process analysis provides the input data for the techno-economic analysis of all fuels, which was also carried out in the C3 mobility project, in the form of a detailed list of components for the production of methanol. With the results of work package A, the map shown in Figure 2 for the power-to-fuel (PtF) efficiency of methanol synthesis was created. This PtF efficiency describes the ratio of the energy bound in the synthetic fuel to the energy expenditure of its production, i.e. the production of hydrogen (electrolysis), the provision of the required carbon dioxide, and other consumables such as electricity or steam. The results in Figure 2 show that both the type of electrolysis and the CO2 source have a significant influence on the overall efficiency of the methanol synthesis. PtF efficiencies of 50-60% can already be achieved today with established CO2 separation processes available on the market, such as MEA amine washing and electrolysers (AEL / PEM). With a forecast development in electrolysis due to increasing demand, PtF efficiencies of over 60% will also be possible in the future.